‘The importance of symbiosis in philosophy of biology: an analysis of the current debate on biological individuality and its historical roots’
- 411 Downloads
- 1 Citations
Abstract
Symbiosis plays a fundamental role in contemporary biology, as well as in recent thinking in philosophy of biology. The discovery of the importance and universality of symbiotic associations has brought new light to old debates in the field, including issues about the concept of biological individuality. An important aspect of these debates has been the formulation of the hologenome concept of evolution, the notion that holobionts are units of natural selection in evolution. This review examines the philosophical assumptions that underlie recent proposal of the hologenome concept of evolution, and traces those debates back in time to their historical origins, to the moment when the connection between the topics of symbiosis and biological individuality first caught the attention of biologists. The review is divided in two parts. The first part explores the historical origins of the connection between the notion of symbiosis and the concept of biological individuality, and emphasizes the role of A. de Bary, R. Pound, A. Schneider and C. Merezhkowsky in framing the debate. The second part examines the hologenome concept of evolution and explores four parallelisms between contemporary debates and the debates presented in the first part of the essay, arguing that the different debates raised by the hologenome concept were already present in the literature. I suggest that the novelty of the hologenome concept of evolution lies in the wider appreciation of the importance of symbiosis for maintaining life on Earth as we know it. Finally, I conclude by suggesting the importance of exploring the connections among contemporary biology, philosophy of biology and history of biology in order to gain a better understanding of contemporary biology.
Keywords
Symbiosis History of biology Philosophy of biology Biological individuality Hologenome Holobiont Units of selectionThat symbiosis is a universal phenomenon in our planet is something that does not escape the attention of biologists. Organisms of different species constantly engage with each other in various types of associations, amongst which symbiosis–the persistent relationship among individuals of different species (Paracer and Ahmadjian 2000)– stands out as an essential phenomenon for the maintenance of life on Earth as we know it. For instance, it is widely acknowledged that the bodies of most animals contain an important number of bacterial partners, which sometimes even leads to the duplication of the number of their own cells (Huttenhower et al. 2012; Relman 2012; McFall-Ngai et al. 2013; McFall-Ngai 2015). Furthermore, the important role of symbionts for the physiology and normal development of their hosts is generally recognized and widely supported by current biological evidence (Gilbert and Epel 2009; Brucker and Bordenstein 2012, 2013; Sommer and Bäckhed 2013; Rosenberg and Zilber-Rosenberg 2014; McFall-Ngai 2015). Finally, the importance of symbiosis in some events of speciation has been recently explored and it is currently gaining empirical support (Jaenike et al. 2010; Brucker and Bordenstein 2012, 2013; Gontier 2015; Lipnicki 2015).
The acknowledgment of the importance of symbiosis for the maintenance of life on Earth, as well as the universality of the phenomenon, has recently led philosophers of biology to question the definition of some of the most important concepts in the field. Particularly important, the monogenetic uniqueness of organisms as well as the boundaries between organisms and their environment have been questioned, challenging some of the traditional definitions of biological individuality. Should the symbiotic microorganisms that reside within the bodies of animals and plants be considered parts of a holistic unit that encompasses the host and its symbionts or, on the contrary, should those microorganisms be considered independently from the host? If they should be considered parts of the host, forming a higher level entity, what is the metaphysical status of this higher level entity? Is it itself a biological individual, or an ecological community of different independent individuals? Can symbiotic assemblages be considered units of selection, i.e. objects that form populations that evolve following Darwinian dynamics? If so, how does this affect the concept of heredity? And how do symbiotic associations evolve through time? As McFall-Ngai et al. have summarized: “[t]hese new data are demanding a re-examination of the very concepts of what constitutes a genome, a population, an environment, and an organism” (McFall-Ngai et al. 2013: 3234).
Those basic philosophical questions are not completely new, and their origin can be traced back in time to the first moments in which symbiosis thinking began to flourish, and the definition of the concept was not clear.1 For instance, at the end of the nineteenth century a popular trend in biology started identifying symbiosis with “mutualism” (Martin and Schwab 2012). This attitude led those authors to emphasize the existence of a “shared dependency” among partners (physiological, morphological), in which the biological individuality of these partners might be sacrificed in benefit of the “bigger entity”. This, for example, was the position of Albert B. Frank in his early research on mycorrhizas in 1885 (Frank 1885 [Frank 2005]; Trappe 2005). However, if on the contrary most symbionts are interpreted as parasites, this would led to emphasize the individuality of the symbionts that engage in the relationship, suggesting that they will engage in the relationship for their own benefit, not losing their autonomy. This position was vigorously defended by Roscoe Pound (1893). Their disagreement suggests that the conception of symbiosis that one holds has consequences for how to conceive biological individuality.
This review has two purposes: first, it aims to analyse the influence of symbiosis thinking in recent philosophy of biology, particularly reflecting how it has influenced the debates about the boundaries and constitution of the biological individual, as well as the debates about the units of selection; second, it aims to uncover the historical roots of the relation between the concept of symbiosis and the philosophical controversy about what constitutes a biological individual.2
The review will be divided into two parts. The first part, historically oriented, will introduce the concept of symbiosis and analyse its conceptual evolution since it was first proposed by Anton de Bary in 1879. The emphasis of this section will be put in how the concept of symbiosis did already question, since its original formulation, the boundaries and the constitution of biological individuals. The second part of the paper will be centred on recent developments in microbiology, paying special attention to the hologenome concept of evolution, and how those developments have affected current debates on the notion of what constitutes a biological individual, as well as its connection with the debate about the units of selection. I argue that most of the philosophical issues raised by the hologenome concept of evolution were already present in the original debates about symbiosis, and I try to uncover their historical roots, drawing four parallelisms between past research on symbiosis and the research done in the light of the hologenome concept. Finally, I suggest that the recent awareness of the philosophical significance of symbiosis originates from three facts: first, the appreciation on the universality of the phenomenon, which derives from the development of new techniques to identify the presence of microorganisms in the body of multicellular organisms (microbiomics); second, its importance for sustaining life as we know it, including the role that symbionts play in the physiology and development of multicellular organisms; third, the consideration of some symbiotic assemblages –holobionts– as units of selection, which caught the attention of philosophers who were previously not so interested in the phenomenon of symbiosis.
1 Part I. The historical roots of the concept of symbiosis – philosophical implications
This part of the review explores four main ideas relating to the concepts of symbiosis and biological individuality. The genesis of these ideas will be traced back to the authors that first proposed them. I will begin by considering the work of Anton de Bary, who first considers symbiosis as a separate biological phenomenon, naming it, and characterizing its specific properties. At this time, I will argue, symbiosis was already understood to challenge received ideas on the physiological boundaries of the individual; and yet, symbiosis was not then clearly distinguished from other phenomena, e.g. biological “sociality” (section 1). Second, I will consider Roscoe Pound’s criticism of a symbiosis understood as mutualism, and I will introduce the arguments he presents to justify his opposition, paying special attention to his reliance on the concept of “struggle for life” (section 2). Third, I will analyse Albert Schneider’s “The Phenomena of Symbiosis”, as the first systematization of the concept and, more importantly for the purposes of this paper, the first moment in which symbiosis was understood as a phenomenon that might evolve over time, and which could be analysed independently of the organisms that interact symbiotically. I argue that Schneider supposes the first important step in considering symbiotic assemblages as units of selection (section 3). Finally, I argue that the last step for considering symbiotic assemblages as evolutionary individuals (i.e. as questioning the conventional frontiers of the evolutionary individual, of the entity that “struggles for life”) was accomplished by Constantin Merezhkowsky,3 when he hypothesized about the symbiotic origin or chloroplasts, thus creating the conceptual possibility of imagining a hereditary symbiosis (section 4).4
1.1 Anton de Bary (1831–1888)
The introduction of the term “symbiosis” in biology is usually credited to Anton de Bary, who originally used it for the first time in the history of biology in his speech to the Association of German Naturalists and Physicians, “Die Erscheinung der Symbiose”, the “phenomenon of symbiosis” (de Bary 1878 [2016]; Oulhen et al. 2016).5 Nonetheless, one year before de Bary’s lecture, Albert B. Frank had introduced the term “Symbiotismus”, to designate those “cases where two different species live on or in one another” (Frank 1877, quoted in Sapp 1994: 6). When they first used the term, both Frank and de Bary were interested in the study of lichens, whose dual nature had been hypothesized ten years before by Simon Schwendener (Honegger 2000; Egerton 2015; Gontier 2016a). For Schwendener, the dual nature of lichens was understood as a relationship where the fungus is in control of the algae, that it uses to obtain its nutrients, but also, and most importantly, as a new biological individual: “the organisms are so intrinsically and reciprocally connected that through their penetration and merging, they constitute new plants with a clear individual character” (1868, quoted in Gontier 2016a; emphasis added). De Bary, drawing upon those observations plus the experimental results that demonstrated that the dual nature of lichens was not merely a fiction of Schwendener –the two elements that constitute the lichen were separated for the first time in 1876, and by 1877 it was already possible to synthetize lichens in the lab by merging algae with fungal spores (Stahl 1877, referred in de Bary 1878 [2016]; Sapp 1994; Egerton 2015: 104, 106; Gontier 2016a: 276)–decided to refer to “the living together of differently named organisms” by the term “symbiosis” (1878 [Oulhen et al. 2016: 133]).
In his original lecture, de Bary emphasizes two aspects of symbiotic relationships: first, the different degrees of dependency that the partners in a symbiotic relationship sometimes generate with respect to each other; second, the different kind of effects that can be generated as a consequence of the symbiotic association. With respect to the latter point, by the time when de Bary coined the concept of “symbiosis”, Pierre-Joseph van Beneden’s classification of the different types of associations between organisms in mutualism, commensalism and parasitism, had become very popular (van Beneden 1876), and de Bary would precisely use that classification to better capture the nature of symbiotic phenomenon, a phenomenon of which, in his words: “[p]arasitism, mutualism and lichenism are special cases” (1878 [Oulhen et al. 2016: 136]; emphasis added).6 With respect to the former, de Bary’s speech is predominantly dedicated, in almost its totality, in explaining the types of dependencies among partners, including, especially, the morphological and physiological effects that symbiosis can cause the individuals that are interacting symbiotically. Interestingly, he decided to include lichenism as a distinct type of symbiotic relationship together. Why, then, is lichenism different to mutualism and commensalism?
Most of de Bary’s paper is dedicated in explaining the association between Azolla and Anabaena, Nostoc and Cycas, and the fungi and algae that constitute lichens. In fact, de Bary seemed to perceive something particular in those associations, which is the reason why he asserted:
“When we observe more closely the phenomena described above, we find in the azollas and the cycads as well as in lichens, intimate associations of different species but never an organization that fits one of the categories described at the beginning of this study. For the reasons that I have already explained [see below], we cannot strictly speak of commensalism or parasitism” (1878 [Oulhen et al. 2016: 135])
Furthermore, he also discards the hypothesis that those associations might be considered simply as cases of mutualism: “[i]t is however doubtful that there are mutual advantages to the partners. We can definitely say that they do not harm each other significantly (…). But presently, we have no evidence of the mutual benefits that they could afford each other” (1878 [Oulhen et al. 2016: 136]). What de Bary finds particularly noticeable about the cases of lichenism are precisely the morphological and non-pathological effects of these types of associations, as well as the sorts of physiological dependencies that emerge from the partners. Drawing directly upon the experiments carried out by Stahl (1877), he highlights the important morphological changes that accompany the synthesis of lichens: “right after their association with the fungus of the lichen, the cells of the algae become much larger, contain more chlorophyll, [and] are stronger in every way. Beyond doubt, according to data, that have been known for a long time regarding the structure of the lichen, all of these characteristics are retained for the entire life cycle of the lichen, sometimes for several dozen years” (1878 [Oulhen et al. 2016: 138]). It is precisely at this place of his discussion, when de Bary applies Darwin’s theory, explaining that symbiosis might work as an inducer of the morphological changes that are required for natural selection to generate adaptation (see also Sapp 1994: 9; Sapp 2003: 234–251).
What it is at stake in de Bary’s discussion of lichenism is also a debate about the nature of biological individuality. If the elements that conform the lichen are dissociated, the lichen does not exist anymore, and we would only have a fungus –that will eventually die– and an alga. However, if we put them together to generate new lichen, they become somehow de-naturalized, they lose their main morphological characteristics, adopting a new configuration that makes them different from their free-living counterparts. De Bary’s insistence in the non-parasitic, non-mutualistic nature of lichens is also noteworthy. On the one hand, he seems to have identified a new dimension of the “living together”, which was not reducible to van Beneden’s categories. On the other hand, he still wanted to keep the concept of “symbiosis” to name the associations that, in his words “we can group under the term sociability” (1878 [Oulhen et al. 2016: 136]), including some associations that do not question the individuality of the partners involved (e.g. pollination). Jan Sapp argues that “[t]his was a strategic argument that was designed to ensure that lichens were not discarded as exceptions” (1994: 9). I agree with him, and I think de Bary actually believed he was identifying a very distinct phenomenon, which questioned the conventionally accepted boundaries of biological individuals.7
1.2 Roscoe Pound (1870–1964)
After de Bary delivered his lecture, research on symbiosis started growing and new cases were discovered: Karl Bradt discovered the presence of the symbiotic alga Zooxanthella in the bodies of Hydra and sponges (1881, in Sapp 1994: 11) and Patrick Geddes discovered the presence of non-pathogenic alga in sea anemones (1882); some time later, Albert B. Frank discovered the presence of fungi in the root of legumes, which, he hypothesized, was a symbiont with important physiological functions, naming it “mycorrhiza” (1885 [Frank 2005]). In this period, symbiosis practically became identified with mutualism to a point where the two terms became interchangeable, while the general meaning of which de Bary had suggested became lost (Sapp 1994: 18–34; cf. Martin and Schwab 2012, who argue that the association between symbiosis and mutualism lasted until 1970).8
It is precisely in this context that Roscoe Pound lectured his: “Symbiosis and mutualism,” with the aim of disentangling the two concepts (Pound 1893). Pound started his paper by distinguishing three types of relationships between hosts and parasites: those where the host kills the parasite; those where the parasite kills the host; and those where “the host lives on side by side with the parasite indefinitely” and continues “[a] further development is attained in cases where the parasite and host not only live together, but are mutually beneficial, and, perhaps, even, in extreme cases, inter-dependent” (1893: 509; emphasis added). For him, following de Bary, symbiosis just meant “living together for a long time,” and mutualism is just one of the forms that this “living together” might take. Yet, in most circumstances, he claims, this “living together” does not take the form of mutualism. Furthermore, he provides some clarification that is especially important for the debate about biological individuality: acknowledging that mutualism can take forms other than “living together,” he says “it should be noted that the mutualism of which we are here speaking is mutualism of parasite and host –not mutualism of independent organisms” (1893: 509; emphasis added).Why that distinction between cases of inter-dependent organisms versus cases of independent organisms? What makes the former cases so special? We must recall that de Bary had explicitly said that he had no objection to using “symbiosis” to refer to those associations that can be grouped “under the term sociability.” My impression is that Pound had already perceived the qualitative difference between those two types of association: whereas the latter do not compromise the concept of biological individuality, as the organisms that interact can be clearly recognized as independent, the former do, in so far as the organisms (1) live in close association during all their life cycle and (2) might become inter-dependent to such a agree as to form a new entity.
Granted, there is a qualitative difference between the two types of associations (i.e. sociability vs. symbiosis) in so far as the latter, but not the former, compromise currently accepted ideas about biological individuality. In particular, it challenges the idea that one individual can belong to only one species classified according to the criteria of systematists. The rest of Pound’s paper is dedicated in arguing that the biologists of his time had tended to overemphasize the presence of mutualism, claiming to have identified it in many symbiotic associations, where it was not at all clear that the partners were acting mutually. First, he argues, mutualism does not occur in every lichen: it does not exist in homoeomerous lichens, or in what he calls “pseudo-heteromerous lichens,” although evidence suggests that it might exist in heteromerous lichens, as they exhibit a complex interdependence among the fungus and the algae that form the lichen (Pound 1893: 511–513). Second, he analyses Frank’s studies on mycorrhizas and, while recognizing part of Frank’s discoveries, he refers to some evidence by R. Hartig, “a more sober and trustworthy writer than Frank” (1893: 516). He argued that:
“Organisms are not given to gratuitously assisting one another. Mychorhiza [sic] undoubtedly exists (…). But that there is, in any of these cases, more than the ordinary symbiosis of parasite and host, has not been shown and is improbable. That every tree has its root system covered with mycelia, proves nothing. Every tree has its bark covered with lichens, its twigs with black fungi, and its leaves with parasitic fungi of every description.” (1893: 516)
Finally, he considers the presence of Rhizobium in the root of Leguminosae.9 He says that the evidence is uncertain, and although it might sometimes seem as if the Rhizobium were mutualists, “[t]he bacteria (…) are parasites. They are there for their own purposes, and are incidentally beneficial to the plant” (1893: 518). Moreover, while admitting that in some cases the symbiosis might lead to a mutualism –as the plants infected do better than those uninfected–he continues diminishing the evolutionary importance of these symbioses by criticizing some of Frank’s observations:
“To these probabilities, Frank adds certain characteristic improbabilities. (…) [T]hat the plant develops tubes or hyphae for the purpose of self-infection which it sends through its tissues. (…) [T]hat the roots of the Leguminosae possess the power of attracting Rhizobia, due, as he considers, to some secretion. This is too much for his followers, and I think all will agree that it is the last straw of an unsupportable load with which he has already burdened our credibility.” (1893: 519)10
And he concludes his paper saying:
“Ethically, there is nothing in the phenomena of symbiosis to justify the sentimentalism they have excited in certain writers. Practically, in some instances, symbiosis seems to result in mutual advantage. In all cases it results advantageously to one of the parties, and we can never be sure that the other would not have been nearly as well off, if left to itself.” (1893: 520)
Even despite Pound’s dismissal of the importance of mutualistic symbiosis, as well as its general importance, his example helpfully illustrates the general awareness of the phenomenon among biologists in the late nineteenth century. Especially remarkable is his insistence of distinguishing between those cases where interdependence is generated versus those where two (or more) individuals can be recognized as different. Second, and also remarkable, is his way of neglecting the individuality of the symbiotic aggregate. As he expresses here and there, even if in some rare cases the individuality of the symbiotic aggregate might occur, the organisms are there for their own benefit, and many of them would probably be better outside the symbiosis.11 These claims have two important consequences. First, it suggests that the general rejection of symbiosis research by biologists writing at this time was for the reason that it seemed to negatively affect the traditional conception of biological individuality and “struggle for life” (see also Sapp 2003, 2004). Second, it paves the way for a new and important conceptual change in symbiosis, the important division between symbiosis and other forms of sociality, forms that de Bary had considered as manifestations of the same phenomenon.
1.3 Albert Schneider (1863–1928)
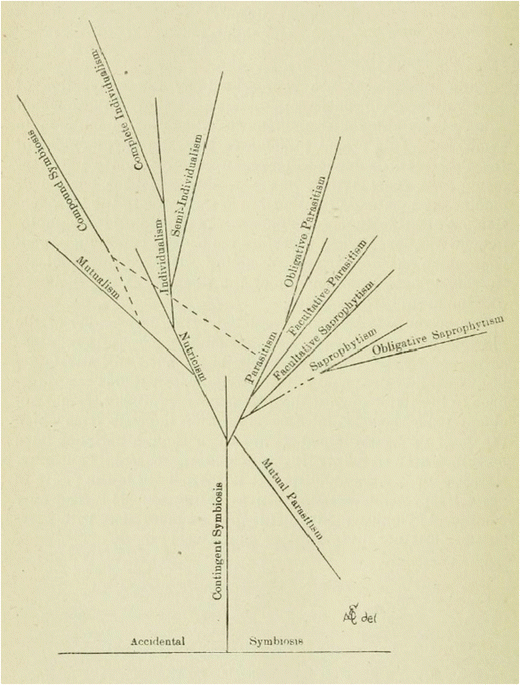
The next important step in the development of the concept was because of Albert Schneider, who in “The phenomena of symbiosis” proposed a new understanding of the symbiosis as “a continuous association of two or more morphologically distinct organisms, not of the same kind, resulting in a loss or acquisition of assimilated food-substances” (1897: 925). There were three purposes to his paper: first, to distinguish clearly between cases of associations of living thing and cases of real symbiosis; second, to suggest the possible evolutionary origin of symbiosis, accounting for the default behaviour of organisms, which he understood as a “struggle for life”; and third, to classify different types of symbiotic associations. Of course, the three questions are closely connected to one another: once symbiosis is distinguished from mere “association,” the classification of different types of symbiotic phenomena will be partially based on evolutionary criteria. Therefore, the different types of symbiotic relationships will be distinguished by degrees, from the forms that entail independent individuality of the organisms that interact, to those where the associated organisms lose their individuality and merge to form a higher level entity.12
- I.
“Incipient Symbiosis (Indifferent Symbiosis)
- 1.
Accidental Symbiosis
- 2.
Contingent Symbiosis (Raumparasitismus)
- II.
Antagonistic Symbiosis
- 1.
Mutual Antagonistic Symbiosis (Mutual Parasitism)
- 2.
Antagonistic Symbiosis (Parasitism)
- a.
Obligative Antagonistic Symbiosis
- b.
Facultative Anatagonistic Symbiosis
- 3.
Saprophytism
- a.
Facultative Saprophytism
- b.
Obligative Saprophytism
- III.
Mutualistic Symbiosis
- 1.
Nutricism (Semi-Mutualistic Symbiosis)
- 2.
Mutualism
- 3.
Individualism
- a.
Semi-individualism
- b.
Complete individualism” (1897: 930–931)
Phylogentic development of symbiosis attending to physiological criteria. Although saprophytism is included in the figure, showing its relation to other symbiotic forms, it is not classified as a symbiotic relation, according to Schneider; rather, he provides it as a point of comparison
Schneider acknowledges from the beginning of his paper the difficulty of determining the starting point of the symbiosis. Under the heading of “incipient accidental symbiosis” he includes those cases where the organisms are in close physical contact for a sufficiently prolonged time, understood ontogenetically, and irrespectively of whether or not morpho-physiological changes (either antagonistic or mutualistic) occur.13Moreover, he argues that, once an accidental symbiosis has been established, then the condition will immediately be subject to change, since the permanency of this symbiosis is in direct proportion to the degree of mutualistic specialization (1897: 934). In this sense, if an accidental symbiosis is not broken down, it will evolve towards a “contingent symbiosis,” where the organisms involved, despite not experiencing any morpho-physiological change, seem to manifest a sufficient degree of elective affinity. One case of contingent symbiosis, according to Schneider, is the bacterial flora of humans, which shows a certain degree of elective affinity but does not seem to show any kind of morpho-physiological relationship with the host.14
The second symbiotic phenomena considered are the cases of “Antagonistic Symbiosis.” According to Schneider, this category includes “mutual parasitism,” i.e. the situation where both organisms live together but their relationship is mutually damaging, “parasitism,” a situation where one of the organisms is damaged whereas the other obtains benefit from the relation, and, as a limiting non-symbiotic case, “saprophytism”. For Schneider, antagonistic forms of symbiosis can only give rise to very limited morpho-physiological specializations or adaptation, since the parasitic nature of the relationship causes it to be “a destructive association, [such that] [t]he morphological and physiological changes tend towards dissolution rather than evolution” (1897: 936). Therefore, he has a reason to believe that, even if antagonistic forms of symbiosis are conceivable (and, in principle, even expectable), a case of antagonistic symbiosis either evolves towards a case of mutualistic symbiosis or it will be driven towards extinction.15
The final kind of symbiotic phenomena, the cases of “mutualistic symbiosis,” can occur when two organisms interact with each other so that the relation is mutually beneficial. The mutual benefit might occur either because one organism benefits another without being damaged (“nutricism”), because both organisms “mutually benefit each other [while] are still capable of leading an independent existence” (“mutualism”) (1897: 941), or because “one or more of the symbionts is absolutely dependent upon the other for its existence” (“individualism”) (1897: 943). Schneider remarks, however, that it is very unlikely that something such as “absolute nutricism” really occurs in the biological world. He acknowledges that in some symbiotic associations one of the symbionts is clearly benefitted, whereas the material benefits for the other are not so clear. However, he thinks that in most cases nutricism will tend to evolve towards a relation of mutual benefit for both partners. This last type of relationships might happen either in cases where both symbionts can carry independent existence (he mentions insectivorous plants and their bacteria, Actinia prehensa and Melia tessellata or some species of ants and the branches of trees), or in cases where they are mutually dependent. About this last case he claims “[i]t (…) represents a higher form of mutualism, from which it is no doubt phylogenetically derived. (…) [In individualism] [t]he associations form an individual, a morphological unit, and the phenomena are frequently not recognized as symbiosis” (1897: 943, emphasis added).
It is important to realize, at this point, that Schneider’s work is conceptually revolutionary. First, he is the first to consider the possibility of studying the phylogenetic history of symbiotic associations (i.e. their evolution): (1) irrespectively of the evolution of the organisms that form the symbiosis; and (2) relative to the opportunities that the environment offers for their evolution (in this sense, symbiotic assemblages would be something conceptually similar to what we now call “units of selection”). Second, he realizes that symbiotic associations challenge the individuality of the organisms that interact, to the point that they might become a new independent emergent individual. Conceptually speaking, Schneider is the first author to recognise this last fact, thus opening the possibility of understanding symbiotic associations as genuine evolutionary individuals in their own right. Furthermore, he is conscious of the physiological importance of symbiosis, as well as why it is occasionally not possible to understand the physiology of the organisms in isolation from their symbionts.16 This fact has gained a lot of attention recently, especially after the hologenome concept of evolution was proposed.
1.4 Constantin Merezhkowsky (1855–1921)
A final and important step in the conceptual development of the association between symbiosis and biological individuality is because of the work of Constantin S. Merezhkowsky. In his “Über Natur und Ursprung der Chromatophoren im Pflanzenreiche,” (“On the nature and origin of chromatophores [plastids/chloroplasts] in the plant kingdom”), Merezhkowsky proposed, for the first time, the term “symbiogenesis,” further advancing the conception of symbiosis as an evolutionary mechanism (Merezhkowsky 1905, 1910) (see also Khakhina 1992; Sapp 1994: 47–59; Martin and Kowallik 1999; Sapp et al. 2002: 418–423; Gontier 2016b).
In his paper, Merezhkowsky aimed at discerning the origin of chloroplasts in plants. During his time, it was commonly believed that the chloroplasts, contained in the body of plants, appeared de novo every new generation and, furthermore, that they originated autogenously, as new organs which differentiated within the bodies of plant cells. Merezhkowsky strongly disagreed with that conception. Drawing upon Schimper’s discovery that chloroplasts do not appear de novo in plant cells, but are always present within their bodies since the beginning of the life of the plant (1885, referred in Merezhkowsky 1905), he proposed a revolutionary notion: chloroplasts should not be regarded as autogenous organs of plants, but as symbionts, i.e. as independent (foreign) organisms that live together with plant cells. Merezhkowsky offered two different types of arguments to support his theory. His first two arguments were theoretical. The first one was based on Schimper’s discovery: if chloroplasts do not arise de novo, though invaginations of the cytoplasm of the cell, but “rather, they always arise through division of pre-existing plastids, and since the latter in turn arise from pre-existing plastids, etc., we necessarily arrive at the logical conclusion that long ago the first chromatophore migrated into a colourless organism” (1905: 596 [Martin and Kowallik 1999: 289]).17 Secondly, Merezhkowsky argued that chloroplasts can be understood by analogy to Zooxanthella in the body of Amoeba viridis. In both cases, the structures (chloroplasts and Zooxanthella, respectively) can be said survive, divide and behave as independent organisms. If biologists do not have any issue in understanding Zooxanthella as independent symbiotic organisms within the bodies of their hosts, they should not have any prejudice in applying the same type of reasoning to chloroplasts, providing that the empirical evidence supported this conception.
The rest of the rationale to his hypothesis were of empirical observation. First, the discovery that chloroplasts, in contrast with other “organs” (“organelles”) in the body of plant cells, can survive and reproduce even after the nucleus of the cell has been removed, and also can do so outside the cell’s cytoplasm, which suggests that they behave like independent organisms. Second, the similarity between chloroplasts and free-living bacteria, concretely, with free-living forms of Cyanophyceae, which has been qualified by Martin & Kowallik as the “unquestionably most novel line of reasoning” (1999: 287). According to Merezhkowsky, chloroplasts and Cyanophyceae had a very similar physical appearance, both in form and colour, very similar biochemical (physiological) properties (with a similar type of nutrition), and analogous ways of proliferation and reproduction, which “makes it exceedingly likely that chromatophores are Cyanophyceae that invaded the plasma” (Merezhkowsky 1905: 600–601 [Martin and Kowallik 1999: 291]). Finally, he argued that, as it was empirically proven that Cyanophyceae can also engage in symbiotic relationships with other organisms (diatoms, rhizopods, etc.), even with cells that are protected by a cell wall, it was possible that at some point in their evolutionary history Cyanophyceae could have entered in contact with a plant cell so as to give rise to chloroplasts.
Merezhkowsky’s symbiogenetic hypothesis, as well as his arguments, gives symbiotic ideas a new meaning. Authors writing prior to him had discussed the importance of the symbiotic relationship, the nature of the symbiotic relationship, how symbiotic relationships could cause several morpho-physiological changes in biological individuals, etc. However, no one had considered the possibility that symbiosis might be a hereditary phenomenon, i.e. that symbiotic associations might be intergenerationally transmitted (e.g. like gametes passing between germ-line cells). Authors had assumed that genetically heterogeneous organisms reproduced independently, and later would form symbioses. Merezhkowsky, on the contrary, challenged the necessity of this assumption; and, by implication, questioned the boundaries of biological individuals, understood evolutionarily. For instance, it is not just that different organisms engage symbiotically and later their morpho-physiological independence is lost; in the case of plant cells, also their hereditary independence (evolutionary individuality) is lost, as the two previously independent organisms are now inherited exclusively together. In summary, Merezhkowsky includes the main element that was lacking in the symbiosis picture, conceiving, for the first time, the idea of hereditary symbiosis. In one sense, symbiotic assemblages had already been attributed all the necessary elements for being considered units of selection (variance, inheritance, fitness; e.g. Lewontin 1970; Godfrey-Smith 2009). The question afterwards changed this sense, since it now asked us to determine the real importance of heritable symbiosis. Was this just an isolated case special to a different phenomenon or was it general to symbiosis as such?
The notion of hereditary symbiosis was later supported by Hermann Reinheimer, Andrei S. Famintsyn and Boris M. Kozo-Polyansky (1924/2010) (Khakhina 1992; Sapp 1994: 47–59; Carrapiço 2015). Afterwards, Paul J. Portier (1918) and Ivan E. Wallin (1927) would apply symbiogenetic ideas to the origin of mitochondria, extending Merezhkowsky’s original application to another cellular organelle. And even later, Paul Buchner would explore the importance of hereditary symbiosis in insects, proposing a new field of application for the hypothesis (Boucher 1965; Sapp 2002). Symbiogenetic theories of the origin of the eukaryotic cell, however, were frequently rejected. This trend continued for almost 50 years, until Lynn Margulis provided new support and the symbiotic origin of the eukaryotic cell became almost universally accepted (Sagan 1967; Margulis 1970, 1991, 1993; see Sapp 2010). Nonetheless, it is important to remark that the conceptual basis for understanding the role of symbiosis in evolution, as well as the possibility of considering some symbiotic assemblages as what we would call “units of selection” in contemporary jargon, were already settled by Merezhkowsky in 1905. By then, all the conceptual connections between the notions of symbiosis and biological individuality were already present, as well as the conceptual challenges that the former presented for traditional conceptions of the later. In the next part of the paper, I will explore how those conceptual connections have been explored in recent times, especially after the proposal of the hologenome concept of evolution.
2 Part II. Holobionts and hologenomes – contemporary philosophical implications of symbiosis
The previous part of the review has analysed how the concept of symbiosis appeared in biology and how the connections between symbiosis and biological individuality changed and developed. Towards the end of this history, the concept would express itself, in the form of hereditary symbiosis or symbiogenesis, and some biologists postulated it as a mechanism of evolution. This part of the review will examine recent conceptual debates in the symbiosis literature, especially the notion of the holobiont and the hologonome concept of evolution. In section 1, I discuss Lynn Margulis’ introduction of the concept, as well as its relation to Merezhkowsky’s notion of “symbiogenesis”. I argue that Margulis’ view of the holobiont is ambiguous: sometimes the holobiont is apparently restricted to cases of hereditary symbiosis and other times it is not. In section 2, I discuss the hologenome concept of evolution, as Rosenberg and Zilber-Rosenberg introduced it, and I review the current debates that it raises in connection to the problems presented by the concept of biological individuality. In section 3, I relate the hologenome concept of evolution to the historical discussion presented in Part I, arguing that the conceptual disputes that the hologenome concept has generated are not new, but only a progression of the previous disputes that were held in the nineteenth century. I observe four parallelisms which obtain between the past disputes on symbiosis and the disputes raised by hologenomes, as well as three further points of distinction.
2.1 The origin of the concept – the importance of Lynn Margulis (1938–2011)
Lynn Margulis (born Alexander) was a pioneer in the field of symbiosis, to which she dedicated almost 50 years. She is especially known for giving new life to the hypothesis of the symbiotic origin of eukaryotic cells, as well as for her enthusiasm about the importance of symbiosis for life on Earth and evolution (Margulis 1990, 1991, 1998, 2010; Sagan & Margulis 2002; Díaz 2015; O’Malley 2017). Margulis is acknowledged as the first person to introduce the term “holobiont”, which was published in her paper “Words as battle cries – symbiogenesis and the new field of endocytobiology” (1990). In this work, she compares cyclical hereditary symbiosis with meiotic sex. In both of these compared cases of inheritance, she argues that two entities are present, which cyclically recognize each other and merge together for every generation. Moreover, in both of these cases she speculates the presence of mechanisms, which guarantee the integration of these two entities and, also, their subsequent dissociation, resulting in the formation of a new individual. An entity formed of two different gametes is what we call a “zygote,” whereas the entity that results from the merger of two symbionts is what Margulis refers to as “holobiont”, which she recognises as a new individual (1990: 676, Fig. 3). Margulis does not, however, specify which “bionts” should be regarded as part of the holobiont, nor does she explicitly define the term in the paper.
One year later, in “Symbiogenesis and symbioticism”, the first chapter of a book she edited with René Fester, Margulis defines the holobiont as a “symbiont composed of recognizable bionts”, and she defines symbiosis as the physical contact between organisms of different species occurring “throughout a significant proportion of the life history” (1991: 2, Table 1). Again, she does not explicitly specify which bionts should be included in the holobiont. If one follows her definition of life history strictly –“events throughout the development of an individual organism correlating environment with changes in external morphology, formation of propagules, and other observable aspects” (1991: 2, Table 1)– it might be argued that the holobiont would encompass all the bionts that share their lifetime together, irrespective of whether they are inherited or not. Clearly, this conception of the holobiont would be incoherent with the concept she had put forward in her previous (1990), where she seemed to suggest that the holobiont should exclusively include the cases of hereditary symbiosis, in her analogy between symbiogenesis and embryogenesis. This second formulation is reasonable if one takes into account the purpose of the chapter, namely, to vindicate the proposition of symbiogenesis as a way in which new species, kingdoms and taxa could evolve –for instance, she says that “the highest level taxa (…) have evolved by acquisitions of symbionts that have become hereditary” (1991: 11, emphasis added)–. This formulation is also coherent with claims she made in her later writings (Margulis and Fester 1991; Margulis 1998, 2010; Margulis & Sagan 2001, Margulis and Sagan 2002; and also see O’Malley 2017). For instance, in one of her latest paper, where she justifies the historical role of Kozo-Polyansky in introducing the idea of symbiogenesis to biology, she argues for the necessity of genetically distinct bionts reproducing together in order for symbiogenesis to occur. Analysing the association between eels and a specific species of shrimp (cleaning symbiosis), she argues:
“It is symbiosis, but not symbiogenesis. Both partners grow and reproduce separately. Both shrimp and eel can live separately. One sees no obvious novelty generated by this symbiosis; i.e., symbiotic physical association. The relationship between the shrimp and the eel is still a behavioral one” (2010: 1528, emphasis added)
In this vein, one might argue that, as “holobiont” was introduced in comparison to meiotic reproduction, and Margulis discusses it while reflecting the importance of symbiogenesis as an evolutionary mechanism (and evolution requires inheritance), the holobiont is thus the biological individual that includes all those symbionts that are inherited together (organelles in eukaryotes, obligatory endosymbionts in insects, etc.) (O’Malley 2017: 36, for a defence of this interpretation).
This interpretation of Margulis’ understanding of holobionts is not without contestation, though. In the same volume where Margulis published her paper, Maynard-Smith suggests “a Darwinian view of symbiosis” (Maynard-Smith 1991). There, he relates the problem of symbiosis to the problem of the units of selection18 and embeds it in the framework of the theory of evolutionary transitions in individuality that he was starting to develop. According to Maynard-Smith, symbiosis can be understood as an evolutionary mechanism and interpreted in a Darwinian fashion (i.e. with the entities that interact symbiotically being a unit of selection) only if the entities that interact symbiotically are transmitted directly, because “[w]ith direct transmission the genes of the symbionts will leave descendants only to the extent that the host survives and reproduces” (1991: 35). Therefore, as far as the two bionts have their fitness interests aligned, it is expected that those symbionts will tend to maintain a mutualistic relation that, eventually, might make it “reasonable to consider the association as a single unit” (1991: 38). However, in cases of indirect transmission, this possibility is much less likely, and he suggests that the interacting entities should be considered as independent units (of selection).
Maynard-Smith’s paper is relevant because he seems to be discussing Margulis’ liberal views about the power of symbiosis. For him, those cases where symbiosis might be considered to have evolutionary power, in the sense of affecting the role of natural selection, are very limited, and probably precluded only to cases such as cellular organelles, as he suggests at the end of his paper. If this is so, then Margulis’ notion of the holobiont might be interpreted not as constrained exclusively to the cases of the eukaryotic cell, but as including the associations of many different bionts. In fact, this view is endorsed in Guerrero et al. (2013), published two years after Margulis’ death. In that paper, holobionts, considered as autopoietic (self-sustaining) units, are defined as “integrated biont organisms, i.e., animals or plants, with all of their associated microbiota” (2013: 133, emphasis added). In the same place, they also coined the term “holobiome”, referring to “the assembly of genetic information contributed by the animal or plant and its associated microbiota” (2013: 134), and demanding a new look at evolution that would take into account the importance of the host genome plus the genome of its microbiota. They argued this to be a new entity, whose basic interacting elements that would give rise to new species and, in general, new biological variety. At some point of the paper, the authors even endorse the theses that: (1) holobionts are subjected to natural selection; and (2) holobiomes are entities that have been selected due to their selective advantages. Even if the authors do not mention the concept "units of selection", their paper might be interpreted as endorsing the hologenome concept of evolution, thus considering the holobiont, with its hologenome (holobiome), as a possible unit of selection in evolution.
Whether Margulis’ concept of the holobiont has to be interpreted as encompassing only hereditary symbiosis or, on the contrary, encompassing the whole collection of symbionts, and whether she was claiming that holobionts are units of selection or not, it seems clear that her conceptual heritage in the field of symbiosis is very important. She was one of the most vigorous defenders of the role of symbiosis for causing novelty in evolution (Margulis 1998; Margulis and Sagan 2002). Moreover, she coined the notion of the “holobiont”, which is one of the most discussed concepts in philosophy of biology at present. In the next section, I analyse the recent usage of the notion of the holobiont, as well as the criticisms that have been raised against it.
2.2 The hologenome concept of evolution and its critics: a review of current debates
The hologenome concept of evolution19 was originally proposed by Eugene Rosenberg and collaborators (Rosenberg et al. 2007), in their review paper: “The role of microorganisms in coral health, disease, and evolution”, as a generalization of the coral probiotic hypothesis (see Reshef et al. 2006).20 Drawing upon their observations on coral disease, the authors suggested the existence of: “a dynamic relationship (…) between symbiotic microorganisms and corals at different environmental conditions that selects for the most advantageous coral holobiont in the context of the prevailing conditions. By altering the structure of its resident microbial community, the holobiont can adapt to changing environmental conditions more rapidly and with greater versatility than a process that is dependent on genetic mutation and selection of the coral host” (2007: 360).
Moreover, reasoning from the existence of this dynamic relation between the coral host and its microbiota, as well as the knowledge that the possibility such a relation offers for the adaptive evolution of a coral to changing environmental conditions, the authors inferred that the coral holobiont must be a unit of selection, i.e. that it is subjected to the process of evolution by natural selection. Drawing upon the observation that, as it happens in corals, all animals and plants harbour an abundant number of symbiotic microorganisms in their bodies, the authors suggest that we generalise the coral probiotic hypothesis to include every animal and plant. Thus, Reshef et al. proposed the hologenome concept of evolution, the notion that “the holobiont with its hologenome should be considered as the unit of natural selection in evolution, and microbial symbionts have an important role in adaptation and evolution in higher organisms” (2007: 360, Box 2).
Nevertheless, in the original paper, the authors do not specify: the meaning of "holobiont", the meaning of "hologenome", or how their hypothesis could be applied to other model organisms. Instead they briefly justify its appeal on four grounds: first, the universality of symbiosis between animals/plants and microorganisms; second, the existence of phenotypic variance between host species and their microbiota, i.e. the fact that hosts of the same species harbour different microbiotas; third, the different range of effects of the microorganisms on their hosts (parasitism, mutualism, commensalism); and fourth, the possible mechanisms of change for the holobiont (including microbial amplification, microbial acquisition, etc.) (Rosenberg et al. 2007: 360, Box 2). However, the authors acknowledged that their reasons were insufficient to support their generalization of the coral probiotic hypothesis. To overcome this difficulty Zilber-Rosenberg and Rosenberg (2008) would publish “Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution” one year later. Beginning with the acknowledgment that microorganisms have been discovered to play a fundamental role in the life of higher organisms (animals, plants), including humans, the authors introduced their hypothesis with a rhetorical question:. “[i]f microbial symbionts play such an important role in the lives of their eukaryotic hosts, why should they not also play a role in the evolution of these higher organisms?” (2008: 723, emphasis added). Zilber-Resenberg and Rosenberg hypothesized that holobionts (i.e. biological entities composed by a host plus all its microbial symbionts), with their hologenomes (i.e. the sum of all the genetic information of the host plus the genetic information of its symbionts) are units of selection. More specifically, concerning the notion of the holobiont, they explicated that:
“Although much of the important research on symbiosis has been carried out with a small number of model systems involving a single major symbiont, the hologenome theory places importance not only on these major symbionts but also on the enormously diverse associated microbiota, which have only been uncovered in recent years using molecular techniques” (2008: 724)
This last point is particularly relevant because it frames the hologenome concept in a very distinctive way. It is not just that very particular host-microbe associations should be considered as units of selection (e.g. the eukaryotic cell, aphids and Buchnera aphidicola, squids and Vibrio fischeri, etc.). This last proposal would not be so revolutionary, after all. The hologenome concept suggests that one should consider the host, with all its microbes (i.e. the holobiont), as a unit of selection in evolution. Notice that this definition of the holobiont might be contrasted with Margulis’ understanding, which seemed to be limited to cases of hereditary symbiosis, at least according to some interpretaters (e.g. O’Malley 2017). What is the justification that Zilber-Rosenberg and Rosenberg believe to have found for their hypothesis? They claim the existence of four sources of evidence: the observation that all higher organisms associate with microorganisms; the fact that symbionts are reliably transmitted intergenerationally; the fact that symbionts affect the fitness of the holobiont; and, finally, the possibility of generating genotypic variation within the holobiont by changing their microbial composition.
It must be noted that the way in which Rosenberg and Zilber-Rosenberg present the hologenome concept is based on a particular interpretation of the units of selection, according to which two types of questions should be distinguished: first, the question about the interactor, or vehicle, the entity that interacts with the environment as a cohesive whole, in such a way that replication is differential21; second, the question about the replicator, the entity of which copies are made (Dawkins 1976; Hull 1980; Okasha 2006; Godfrey-Smith 2009; Lloyd 2017a). For Zilber-Rosenberg and Rosenberg, the holobiont would be an interactor, a cohesive physiological and metabolic entity, whereas the hologenome would be a replicator (see also Rosenberg et al. 2010; Rosenberg and Zilber-Rosenberg 2014, 2016; Author 2015; Bordenstein and Theis 2015; Shropshire and Bordenstein 2016; Theis et al. 2016).
After Rosenberg and Zilber-Rosenberg proposed their hypothesis, the notion that the holobiont with its hologenome constitutes a biological individual has been defended in different ways by different authors, some of which have interpreted it as a unit of selection. Dupré and O’Malley (2009), and John Dupré (2010, 2012) have defended the notion that the holobiont should be considered as the interactor in evolution, in so far as it is the entity responsible for the differential reproduction of the entities that compose it. The authors do not mention, however, the possibility of conceiving the hologenome as a replicator. Scott F. Gilbert, Jan Sapp and Alfred I. Tauber have suggested that we understand the holobiont as a biological individual anatomically, developmentally, immunologically, physiologically and genetically (Gilbert et al. 2012; also Gilbert et al. 2017; Roughgarden et al. 2017). Lynn Chiu and James Griesemer have separately proposed a concept of the holobiont as a developmental hybrid in which the microbes would act as scaffolds of the individuality of the host (Gilbert & Chiu 2015; Chiu and Eberl 2016; Griesemer 2016, 2017). Lisa Lloyd has suggested an understanding of the holobiont as an interactor, as a reproducer, and as a manifestor of adaptation (Lloyd 2017b; see also Griesemer 2017). Ford Doolittle and Austin Booth have proposed to conceive the hologenome as a functional replicator, i.e. as a network of genetic interaction patterns that can be instantiated across different generations of holobionts (Doolittle and Booth 2017; see also Lemanceau et al. 2017); Suárez (under review) has defended a group-selection interpretation of the holobiont, suggesting that we conceive of holobionts as intergenerationally inherited collections of traits associated to successive generations of a particular host. In so far as holobionts can be considered collections of traits, he argues that they can be conceived of as units of selection. Finally, Ehud Lamm has suggested that holobionts should be understood as “structures of evolution”: “constellation[s] of evolutionary factors and their relations […] [that] provide scientists with a common framework and terminology and [allow them] to elicit research questions and hypothesis that apply to many systems of interest” (2017: 372).
Furthermore, some evidence has been gathered in support of the hologenome hypothesis (e.g. Rosenberg and Zilber-Rosenberg 2014; Bosch & Miller 2016, for general summaries). In a pioneer study on Nasonia wasps, Robert M. Brucker and Seth R. Bordenstein have argued that hybrid lethality among different Nasonia species is caused by a disruption of the relation between their species-specific microbiomes and the host genome, which suggests that the different species represent a coevolved hologenome (2013; cf. Chandler & Turelli 2014 for a response; cf. Brucker & Bordenstein 2014).Their study has prompted an immediate interest in the study of the phenomenon of phylosymbiosis, “the eco-evolutionary pattern, whereby the ecological relatedness of host-associated microbial communities parallels the phylogeny of related host species” (Brooks et al. 2016: 1). Convergent host-microbe phylogenies that support the existence of phylosymbiosis have been found in hominids (Ochman et al. 2010; Moeller et al. 2016). Julia K. Goodrich and collaborators have found some evidence that suggests that the microbiome might be heritable and its composition could be partially determined by the host genome (Goodrich et al. 2014, 2016, 2017; see also Turpin et al. 2016). Finally, Thomas W. Cullen and collaborators have found some evidence that might suggest that the host’s immune system might control microbiota acquisition (Cullen et al. 2015). However, some evidence has also been found that suggests that there are no such tight host-microbiome intergenerational associations. For instance, Eric R. Hester and collaborators have not found evidence that supports inheritance of the microbiome among corals. Instead, they found that the microbiota that associates with a coral species are selected according to functional criteria, and thus there are no intergenerational phylogenetic convergences (Hester et al. 2016). The same results have been found in ruminal ecosystems: even if the hosts of the same species might share a functionally similar microbiota, the specific microbial taxa that they associate with are different. The authors explained the occurrence of this phenomenon with a metaphor: “the players might change but the game remains” (Taxis et al. 2015; Doolittle and Booth 2017 base their account of the holobiont on these results).
The hologenome concept, however, has also been contested by many, who propose that: (1) the holobiont is a sufficiently coherent biological entity for it to be considered an evolutionary interactor (Booth 2014; Queller and Strassmann 2016; Skillings 2016); there is no real empirical evidence supporting the claim that the hologenome can be a replicator or a reproducer, in so far as the fidelity of its intergenerational transmission is very low (Moran and Sloan 2015; Godfrey-Smith 2015; Stencel 2016; Douglas and Werren 2016; Hester et al. 2016; Hurst 2017; Stencel & Wloch-Salamon under review). Detractors of the holobiont concept tend to emphasize the lack of shared interests and unifying mechanisms between the entities that compose holobionts; and, on this basis, they are reluctant to accept the notion that holobionts are units of selection in any of the aforementioned senses.
The claim that holobionts are interactors has been recently disputed by Austin Booth who, emphasizing the fact that the different entities that compose a holobiont can reproduce independently, has argued that “the interactor perspective on holobionts, as currently endorsed, suffers from imprecision. More needs to be said about just what kinds of causal interactions among parts serve to bind independently reproducing populations into interactors” (2014: 670). This notion has also been criticized by David C. Queller and Joan E. Strassmann, who argued that holobiont defenders make an illegitimate inference from physical proximity (symbionts living together) to functional integration (symbionts constituting an interactor): “The holobiont is defined by spatial criteria. There is no reason to believe that spatial proximity necessarily leads to functional integration” (2016: 869). And also Derek J. Skillings has criticized this notion on the basis that the entities that compose the microbiome of a holobiont might change during the host’s lifetime. If this is so, he argues, then there are no criteria of identity to recognize a holobiont as a biological individual (sensu organism or interactor), because the microbial species that compose it are constantly and fluidly changing (Skillings 2016).
In relation to the claim that holobionts are replicators, Angela E. Douglas and John H. Werren have rejected the possibility on the basis that holobionts lack the proper type of intergenerational inheritance (Douglas and Werren 2016). For them, the holobiont can be considered a unit of selection if and only if there is sufficient partner fidelity –“stable association of host and symbiont genotypes across multiple generations” (2016: 2) – among the different species that constitute the holobiont. Otherwise, the entities that compose the holobiont would not have their fitness interests aligned; and thus, selection at the level of the holobiont would be disrupted by selection at lower levels. They concede that very specific and tight host-symbiont associations, under very special circumstances, may qualify as units of selection. However, they are sceptical that the same might be said about all the members of the microbiota: “We do not argue that selection cannot act on the host-microbiome as a unit. We simply argue that the evidence for this is weak, and the conditions necessary for it to occur are unlikely” (Douglas and Werren 2016: 5; see also Moran and Sloan 2015; Hurst 2017). Suárez (under review) has offered a specific reply to this criticism, arguing that their requirement of partner fidelity is unreasonable, since it relies on some assumptions about biological individuality that are disputable (the cooperation/conflict concept of biological individuality). Furthermore, he argues that the same type of assumptions are not applied to other levels of the biological hierarchy (e.g. transitions in evolutionary individuality), which creates a disparity of criteria. Finally, Peter Godfrey-Smith has also criticized the notion that holobionts are reproducers on the grounds of his concept of Darwinian populations (2015). He believes that host-microbe associations can only qualify as units of selection in the situations when the host is able to “kidnap” the reproduction of the microbe, i.e. when host and microbe can only reproduce together as a unit, but not independently from each other, since otherwise the system would be disrupted. He claims this to be true of eukaryotic cells generally. Godfrey-Smith also acknowledges the existence of intermediate reproductive stages (i.e. reproduction partially kidnapped, but with a high degree of independence). In any case, he does not believe that there is any evidence to qualify the holobiont, conceived as the host plus all its microbes, as a unit of selection, because the parts can still reproduce independently of the whole and thus will not have the same interests.
The debates between defenders of the hologenome concept of evolution and its detractors reflect diverging conceptions of biological individuality. Defenders of the hologenome concept tend to emphasize the collaborative nature of life, as well as the importance of symbiotic associations for maintaining life as we know it. They seem to share a commitment to a view of biological individuality according to which the existence of conflicts amongst the parts of a system does not rule out the possibility of the system evolving as a unit. Furthermore, they concentrate on studying symbiosis as an independent phenomenon, and try to understand the evolution of symbiotic relationships by partially abstracting away from the organisms that engage in symbiosis. Detractors of the concept, on the other hand, tend to emphasize the impossibility of having a biological individual if the parts of the systems are in conflict with one another, thus rejecting any claim about the individuality of holobionts. They are prone to consider holobionts as mere ecological communities of independent organisms that are together due to environmental convenience, not due to shared evolution. They put more emphasis on the study of the different species that engage in the symbiosis that in the study of the evolution of the symbiotic relationship itself. More research is needed to determine the empirical consequences of the hologenome concept of evolution, as well as to unravel the empirical consequences of the different conceptual assumptions made by defenders and detractors of the notion. Research on the historical roots on some of the recent debates will help to determine the origins of some of the present assumptions in current debates, as well as help with clarifying different issues raised by the hologenome concept of evolution, some of which were already present in the debates of prior literature.
2.3 The historical roots of the hologenome concept of evolution
Most of the debates about the hologenome concept of evolution explored in the previous section parallel some of the debates about symbiosis explored in Part I. I will explore four parallelisms between them, uncovering the similarities between recent research and the research conducted in the nineteenth century. Finally, I will explore the novelties introduced by the hologenome concept of evolution, exploring its differences to previous research.
2.3.1 First parallelism. The importance of mutual dependence among organisms
One of the aspects of life that symbiosis research has emphasized since it originally appeared was the mutual dependencies that exist among organisms. Without being necessarily mutualists, organisms frequently rely and depend on each other in order to survive and reproduce. De Bary found that some of those dependencies were not just circumstantial, but were maintained throughout the entire life cycle of certain individuals of different species. After de Bary, many other scientists stressed the importance of mutual dependencies among organisms in order to sustain life as we know it. Defenders of the holobiont concept stress the existence of those mutual dependencies among organisms, putting a special emphasis on the interactions of animals and plants with their microorganisms. They frequently insist that the phenotypes of known animals are not the result of a genetic plan that develops without external influences, but are the result of a co-construction between the animal themselves and their symbionts. They stress that anatomically, immunologically, developmentally and physiologically we have never been individuals, if individuality is understood as the linear development of a single genetic plan (Dupré 2010; Sapp et al. 2012).
Like the original proponents of the symbiotic concept, including de Bary, Schneider, and Merezhkowsky, contemporary defenders of the holobiont pay special attention to those processes of co-construction and criticize previous approaches that have tended to diminish the importance of collaborations for essential processes. From a conceptual point of view, defenders of the holobiont are not proposing anything new: the founders of symbiosis research knew already that the long-term ontogenetic interactions of individuals of different species questioned basic ideas about the defining boundaries of the individual. If a biological individual is a functional whole that can survive by its own means to a great extent, then individuals do not necessarily match species, as there could be individuals that are composed by two or more different species that interact to form a cohesive bigger whole (see ft. 5). Therefore, defenders of the hologenome share their ideas with the founders of symbiotic thought; and, in this sense, their theses are not conceptually new.
2.3.2 Second parallelism. Spatial continuity and biological individuality
One of the arguments used by the detractors of the hologenome concept was based on the fact that from the observation that two entities live together one cannot infer that these two entities are a biological individual. This criticism was explicitly expressed by Queller & Strassmann, who denounced the defenders of the holobiont who inferred functional integration of the entities that compose the holobiont from the fact that they live in physical contact (2016: 819). A similar view is expressed by Booth, when he demands the presence of concrete mechanisms that guarantee that the members of holobionts are genuinely bounded together. It is not enough to say that they reside within the body of the host. The type of relationship that those microorganisms maintain with their host needs to be specified, or otherwise their common boundedness would be biologically irrelevant.
Queller & Strassmann’s and Booth’s observations match closely with the observation made by Pound in 1893. Criticizing Frank’s assumptions about the symbiotic character of mycorrhizas, he argued “[t]hat every tree has its root system covered with mycelia, proves nothing. Every tree has its bark covered with lichens, its twigs with black fungi, and its leaves with parasitic fungi of every description.” (1893: 516). His criticism, even if it was directed at a different type of association, rests on the same kind of assumptions about biological individuality. To prove that two entities living together are a biological individual, one needs to prove that there is a shared functionality. Inferring that two entities are a unique individual (or that they relate to each other mutualistically) from the fact that they share the same physical boundaries is insufficient. Therefore, the criticism raised by Queller & Strassmann and by Booth cannot be considered as conceptually novel. It is true that the criticism applies to an entity that, intuitively (i.e. based on physical appearance), might be considered more “individualistic” than the association between mycorrhiza and trees, which Pound discussed. However, this does not mean that the structure of the arguments used to criticise the concept are different.
2.3.3 Third parallelism. Studying the symbiotic phenomenon independently of the organisms that engage in the symbiotic relation
Defenders of the hologenome concept tend to emphasize the functional relations that exist between specific hosts and their microbiota. Different researchers have stressed the importance of a proper and balanced microbiota for the healthy physiology (and development) of organisms. From this observation, many authors have inferred evolutionary consequences, as well as a history of shared coevolution among independent genomes that form a hologenome. In some cases, like in Doolittle and Booth’s (2017), the hologenome has been defined functionally, as a set of functionally relevant genetic networks that are reconstructed again and again in every new realisation of a holobiotic unit. This functional view of the holobiont and the hologenome abstracts away from the organisms that interact symbiotically. What matters is that the same functional relationships reoccur every generation, as well as the evolution of those relationships, irrespectively of the organisms that guarantee that this happens.22 This position contrasts with organism-centred views of symbiosis, in which what is significant is not so much the evolution of symbiosis itself, but the evolution of the organisms that engage in the symbiotic relationship.
Schneider might be taken as a key reference for those positions, in so far as his work emphasized the study of the evolution of the symbiotic phenomenon in itself, irrespectively of the organisms that engage in the symbiotic relationships. As he argued, symbiosis research should study “the phylogenetic relationship of the symbioses without any reference to the phylogeny of the organisms comprising them” (1897: 931). Furthermore, he also emphasized the importance of environmental opportunity for establishing symbiosis. The new functional approach towards understanding symbiosis could be conceptually understood in the terms of Schneider, and it seems significantly connected to his prescriptions about how to study the phenomenon of symbiosis and the different symbiotic relations that exist in nature. In this sense, conceptually speaking, the emphasis on physiology for understanding the nature and evolution of symbiosis is not new at all; it was already present in past literature.
2.3.4 Fourth parallelism. From symbiosis to symbiogenesis. The origin of new individuals through symbiosis
Merezhkowsky famously emphasized the importance of symbiosis as an evolutionary agent that can generate evolutionary novelties (new structures) as well as new biological individuals. In this vein, he was situating symbiosis outside of the realm of ecology and putting it in the realm of evolution. Even if the importance of symbiosis for evolution had been also emphasized for other authors (de Bary, for instance, who pointed out the possibility of using symbiosis for doing evolutionary experiments and emphasized the importance of symbiosis in creating new biological structures), it was Merezhkowsky who first appreciated the possibility of generating new biological individuals as a consequence of the symbiotic merger of two previously extant ones. Defenders of the hologenome concept have exploited this last possibility and applied Merezhkowsky’s ideas generally, not only to eukaryotic cells. If mitochondria are former symbionts that are now considered parts of a new biological individual (i.e. the eukaryotic cell), so too should the microorganisms that compose an animal’s microbiota be considered parts of a new individual.
Conceptually speaking, there is no big difference between the hologenome concept and Merezhkowsky’s ideas about symbiogenesis. In both cases, it is assumed that new individuals can emerge through symbiosis and that these new individuals will have new biological properties. Furthermore, in both cases symbiosis qua symbiogenesis is put in the realm of evolution, and is not considered exclusive to the realm of ecology. In this sense, the hologenome concept is not conceptually revolutionary, as the ideas were already present in early twentieth century biology. The qualities that differentiate the hologenome concept from previous developments in symbiotic thought, thus, must lie elsewhere.
2.3.5 What is new about the hologenome concept?
-
First, the hologenome concept of evolution appeared after the “omics” revolution, a moment when the technological tools available for scientific research allowed biologists to discover an important number of microorganisms that had been previously unnoticed (Rosenberg and Zilber-Rosenberg 2014). In this sense, and in contrast with previous research on symbiosis, the hologenome concept is more universal, as it departs from the empirical evidence that all animals and plants bear an important number of microorganisms within their bodies. Previous research on symbiosis, however, had not been able to detect the universality of the phenomenon, and only some specific cases of symbiosis were studied. In addition, in previous research the emphasis was put on very specific symbionts, those that reappear across different generations of the same host and play a very specific role during the host lifetime (normally endosymbionts). The hologenome concept, however, changes the focus of the research and extends it to the whole microbiota. In this vein, the foci of the research are not particular host-symbiont associations, but the association between a host and all of its microbes.
-
Second, a fundamental element that frames contemporary discussions about symbiosis is the role of microorganisms for maintaining life as we know it. More concretely, the hologenome concept appeared as a (alleged) conceptual consequence of the observations of the conditions under which healthy corals could grow (i.e. the “coral probiotic hypothesis”). The proponents hypothesized that the best way of explaining health and disease among corals was to propose that corals, with their microbiome, constituted a single unit of selection in evolution. In this sense, the discovery of the physiological relationships between animals and plants and their microorganisms is the basis of the hologenome concept, as well as the basis for understanding its philosophical significance. In fact, this is what distinguishes Schneider’s account of the bacterial flora and the account put forwards by defenders of the hologenome concept: while Schneider recognised the existence of an elective affinity between microorganisms and their host, he believed this to be of reduced significance, and thus situated this as a case of “accidental symbiosis”. In recent years however, it has been shown that the relationship between a host and its microbiota is not just merely “casual”, but that there are very concrete physiological (and developmental) functions that are partially determined and/or realized as a consequence of its presence. This is particularly important because it encourages us to think of the phenomenon’s evolutionary possibility. The hologenome concept of evolution is a hypothesis about why this elective affinity, whichis accompanied by the realization of basic functions could have appeared and evolved through time.
-
Third, the hologenome concept of evolution, in contrast with previous discoveries made for symbiosis, has caught the attention of many philosophers of biology that had previously not considered the symbiotic “habit” in much detail. I think there are two reasons for this. The first reason is that, after the important developments in the “omic” sciences, philosophers of biology started paying more attention to microorganisms (O'Malley 2014). In fact, philosophy of biology has been accused of highly ignoring the importance of microorganisms, which despite constituting about 80% of the total biota had not played a significant role in many philosophical disputes (O’Malley and Dupré 2007). This attitude has changed in recent years, and this change is important if we are to understand why the conceptual problems raised by symbiosis research have become more urgent for the philosophers of biology writing at the present. A second important change is the way in which the defenders of the hologenome concept, especially Rosenberg and Zilber-Rosenberg, have framed the debate. In contrast to previous research on symbiosis, which, while acknowledging the evolutionary importance of symbiosis, still treated the phenomenon in ecological terms, the defenders have put emphasis on the evolutionary importance of symbiosis. Particularly, they have provoked one of the most agitated debates among philosophers of biology, the debate about the units of selection. I believe that the emphasis of their understanding of symbiotic assemblages (holobionts) in terms of the units of selection debate has been of special importance for the engagement of philosophers, who have been discussing the issue of units of selection for about half a century. In this vein, framing the debate in terms of units of selection is conceptually novel in relation to previous (nineteenth century) debates.
3 Part III. Concluding remarks
This paper has reviewed some of the current debates about symbiosis and its relation to the problems of biological individuality. It has also traced the historical roots of the current debates, and argued that some of this arise as a consequence of the hologenome concept of evolution, which was were already present to some extent in the nineteenth century, in the context of the original problem of explaining the “living together” of individuals. The review shows how current biological disputes are partially grounded in different philosophical assumptions, but concretely grounded in different conceptions about biological individuality. I have argued that defenders of the hologenome concept tend to emphasize the collaborative aspect of life, and that they show a tendency to focus their studies on the evolution of the symbiotic relationship, irrespective of the different organisms that engage in the symbiosis. On the contrary, detractors of the hologenome concept tend to emphasize the conflicting interests of the entities that compose the holobiont, and, on these grounds, tend to reject any attribution of individuality, conceiving the holobiont as a community of relatively independent individuals. The disagreement among both parties in the dispute is based upon diverging conceptions about biological individuality, as well as upon diverging conceptions about the focal unit of analysis.
Finally, the review has also revealed the connection between the original debates about symbiosis and contemporary debates. I have drawn four parallelisms between the historical and contemporary debates, and emphasized three distinctive issues of the current debates. I have shown how the disagreements amongst both the defenders and the detractors of the holobiontare similar to some of the disagreements of both the defenders and the detractors of symbiosis during the concept's modern inception. In general, the review has shown the existence of an intimate connection between biology, history and philosophy, and how different philosophical assumptions might underlie current debates in biology. Furthermore, I have suggested the importance of the relationship between philosophy and current biological thought, especially concerning the debates on biological individuality, the holobiont and the units of selection, and I have emphasised the historical origin of these debates. I suspect that many current debates in biology are also affected by diverging philosophical assumptions, which have their specific historical background also. Studying these assumptions, as well as their historical sources, is an important and constructive task facilitating firther clarity and understanding on some of these contemporary debates. In this sense, biology, philosophy of biology and history of biology, far from being completely separate disciplines, are totally entangled with one another.
Footnotes
- 1.
- 2.
The review is not about the problem of biological individuality and how different biologists and philosophers have conceived the topic; rather, this review is about the relation between symbiosis and certain dimensions of the problem of biological individuality –the boundaries and composition of the biological individual and the units of selection. However, the reader must at least take into account that three different notions of biological individual will be considered, especially in part 2: biological individuals as functionally integrated units, biological individuals as units of selection and biological individuals as bounded units (with clear physical boundaries, such as a membrane). Readers interested in the philosophical problem of biological individuality might refer to Wilson and Barker (2013), Bouchard and Huneman (2013), Pradeu (2016a), DiFrisco (2017) and Lidgard & Nyhart (2017: 17-63).
- 3.
There are alternative ways to spell his name (e.g. Merezhkovski, Mérejkovski, Mereschkovsky). I use the spelling that appears in Sapp et al. (2002).
- 4.
My historical focus is selective and not exhaustive, since I aim to compare four parallelisms between the historical development of the concept of symbiosis and the recent developments of the concept of holobiosis. The reconstructed history I will present will reflect this interest. For the readers who are interested in seeing different historical reconstructions see Sapp (1994), Paracer & Ahmadjian (2000: 231-238), Wilkinson (2001), Peacock (2011), Martin and Schwab (2012), Egerton (2015), Carrapiço (2015), Gontier (2015, 2016a), Zook (2015).
- 5.
Frank N. Egerton, however, in his review paper on the history of symbiosis studies dedicates the first section to studies of symbiotic phenomena that appeared before the concept of “symbiosis” was introduced (2015: 81–90). He goes as far as to Herodotus, Aristotle and Theophrastus. Despite the interest of their research, as far as this review is about the philosophical implications of the concept and its relation to other philosophical concepts, I have chosen to begin with de Bary’s account.
- 6.
Parasitism was known while before van Beneden, but parasites (including those that we might call nowadays microorganisms, Pasteur’s germs) were basically considered as pursuing their own interests, thus necessarily damaging the other in a context of struggle for life (e.g. Spencer 1899; cf. Sapp 1994: 25–28). Precisely, what is innovative about van Beneden’s work was that he was the first in: (1) identifying the existence of an important number of associations among organisms that are not parasitic, a discovery that of course had historical precedents; (2) classifying the different types of biological associations in virtue of their effects in a systematic way, which is also conceptually different from previous views on the economy of nature (Egerton 2015: 84).
- 7.
It is important to note that lichenologists originally rejected Schwendener’s dual hypothesis (e.g. Crombie 1886), denying in some cases the evidence, among other reasons because its acknowledgment would threaten “the hard-won autonomy of lichenists themselves” (Sapp 1994: 4), in so far as lichens would stop being an independent biological individual. Interestingly, lichenologists did not lose their autonomy and it was precisely the study of lichens as dual individuals that began challenging traditional ways of understanding biological individuals more generally. This is the first moment, to my knowledge, that the problem of symbiosis and the philosophical problem of biological individuality get engaged in a way that questions the traditional conception of what counts as a biological individual.
- 8.
One of the reasons why symbiosis became identified with mutualism during this period is related to the influence of the political ideas of the time, especially the anarchist ideas of Kropoptkin (1902). Readers interested in the influence of political ideas on symbiosis thinking can refer to Sapp (1994: 18-25) and Gontier (2016a).
- 9.
Although he did not call them Rhizobium, but “tubercles,” stating “For all that I have read and seen, I am satisfied that the parasites [in Leguminosae] are bacteria, and I see no reason for separating them from the rest of Schizomycetes as Schneider does. I even doubt the necessity of creating a separate genus for them, as Frank did in 1890, under the name of ‘Rhizobium’” (Pound 1893: 517).
- 10.
See Oldroyd (2013) to realize that some of Frank’s observations were indeed true and Pound, while having a fair point about the lack of proper evidence for some of Frank’s statements, could have not been more mistaken.
- 11.
Pound’s seems to assume a concept of biological individuality similar to what Queller and Strassmann have recently called the “cooperation/conflict conception” of the biological individual (2009, 2016). For Pound, as it happens for the authors, symbiotic assemblages cannot be considered individuals in the proper sense, as the entities that engage in the symbiosis are in constant struggle with each other.
- 12.
It must be noted, although in passing, that Schneider does not require that the two organisms that engage in symbiosis belong to different species: he only requires that they are morphologically different. That’s why, from his perspective, the mother and the embryo/foetus, the sexual cells that merge to form a zygote or even tumours or cysts would count as cases of symbiosis. This is, I think, different from de Bary’s original purpose –probably that’s why Schneider says that he uses symbiosis “in its broader meaning, not in the sense of De Bary” (1897: 923, fn. 1)–, who seemed to understand symbiosis requiring different species.
- 13.
Schneider acknowledges the problems of this position, which can be criticized on the same basis as Pound had criticized Frank’s account of mycorrhiza –“[t]hat every tree has its root system covered with mycelia, proves nothing” (Pound 1893: 516). However, he justifies his decision by claiming “[f]rom a priori reasoning one is, however, forced to conclude that the first symbiotic activities began with the first contact of organisms” (1897: 933).
- 14.
See part II of the paper for seeing how these sorts of claims are presently unsustainable.
- 15.
Of course, extinction of the symbiotic association, but not necessarily of the partners that interact symbiotically. Remember that Schneider’s paper aims to study exclusively the phylogenetic evolution of symbiotic associations without reference to the organisms that interact.
- 16.
Those readers who are not familiar with the different types of biological individuals (physiological, anatomical, developmental, evolutionary, etc.) can check Gilbert et al. (2012), Godfrey-Smith (2013), Pradeu (2016a, b), DiFrisco (2017). In brief, however, it is important that she notes that not all criteria for classifying biological individuals necessarily led to coincidental classifications and sometimes different criteria overlap. For the overlapping nature of biological classification see Clarke (2010).
- 17.
It is widely acknowledged that chloroplasts are responsible for the green colour of plants.
- 18.
Maynard-Smith does not use “units of selection”, but “units of evolution”, where a unit of selection is whatever entity exhibit phenotypic variation that led to multiplication of the entity within the population (thus being selected for or against), and a unit of evolution is a unit of selection that, furthermore, exhibits heredity (Maynard-Smith 1987). In contrast with Maynard-Smith, I will use “unit of selection” as it is conventionally used, i.e. requiring heredity, variance and fitness/multiplication, and thus meaning what Maynard-Smith means by “unit of evolution” (see Lloyd 2017a, c: 293–297; Gontier 2010, for an analysis of the concept of “unit of selection”)
- 19.
Originally, they referred to it as the hologenome theory of evolution. Later on, they started calling it the hologenome concept of evolution (cf. Gissis et al. 2017: 303–384).
- 20.
A clear antecedent to the hologenome concept is found in Sapp (2003: 234-251, 2004), when he coins the concept of “symbiome”. He defines the symbiome as the entity “comprising chromosomal genes, organellar genes, viral genes, as well as other microbial symbionts, sometimes inside cells and always outside them, functioning across a continuum from parasitism to mutualism, depending on their nature and context (…). Since every plant and animal consists of complex ecological communities of microbes, the symbiome must function as a unit of selection.” (2004: 1047). Nonetheless, Sapp first presents the concept in a section dedicated to developmental symbiosis (Sapp 2003: 235–236), and there is no reason to believe that a developmental organism should be delineated by the same boundaries than a unit of selection (e.g. DiFrisco 2017). The concept of “symbiome”, however, is not as frequent in current literature as the concept of “holobiont” and it has been recently used with two different meanings: first, to refer to the whole set of symbionts that associate with a host, without including the host (e.g. Boucias et al. 2013; Rosas-Pérez et al. 2017); second, to refer exclusively to “the colocalized and coevolving taxa in a given consortium” (Tripp et al. 2017: 552). If we define the concept according to the second formulation, then one might argue either that symbiome = hologenome (if the hologenome is proven to evolve as a single unit) or that the symbiome corresponds to the part of the hologenome that actually evolves as a single unit (e.g. the set of vertically transmitted symbionts). This warrants further discussion, which is, however, outside the scope of this paper. For my present purposes I will restrict the discussion to the concept of the holobiont sui generis.
- 21.
“Such that replication is differential” does not specify which are the entities whose differential replication might be affected by belonging to an interactor. It is conceptually possible that the holobiont is an interactor that promotes a more efficient replication of the different individuals that compose the holobiont (host, microbes of the microbiome), but not of the hologenome.
- 22.
This position is taken to the extreme in Doolittle (2017).
Notes
Acknowledgments
I would like to thank Staffan Müller-Wille, Sabina Leonelli, Caglar Karaca, John Dupré, José Díez, who read previous versions of this manuscript and made helpful comment. Benjamin Smart is especially acknowledged for all his help and detailed comments in the final version of the manuscript. Finally, I would like to thank two anonymous reviewers for their comments, which clearly helped in improving the content and structure of the paper. This work was economically supported by the Spanish Ministry of Education (FFU16/02570) and the Spanish Ministry of Economy and Competitiveness (FFI2016-76799-P).
References
- Booth A (2014) Symbiosis, selection and individuality. Bio Philos 29:657–673CrossRefGoogle Scholar
- Bordenstein SR, Theis KR (2015) Host biology in the light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biol. https://doi.org/10.1371/journal.pbio.1002226
- Bouchard F, Huneman P (2013) From groups to individuals. Evolution and emerging individuality. The MIT Press, LondonGoogle Scholar
- Boucher P (1965) Endosymbiosis of animals with plants microorganisms. Interscience Publishers, New YorkGoogle Scholar
- Boucias DG, Kariithi HM, Bourtzis K, Schneider DI, Kelley K, Miller WJ, Parker AG, Abd-Alla AMM (2013) Transgenerational transmission of the Glossina pallidipes Hytrosavirus depends on the presence of a functional Symbiome. PLoS One 8(4):e61150PubMedPubMedCentralCrossRefGoogle Scholar
- Brandt K (1881) Über das Zusammenleben von Algen und Tieren. Biologisches Centallblatt 1:524–527Google Scholar
- Brucker RM, Bordenstein SR (2012) Speciation by Symbiosis. Trends Ecol Evol 27(8):443–451PubMedCrossRefPubMedCentralGoogle Scholar
- Brucker RM, Bordenstein SR (2013) The capacious hologenome. Zoology 116:260–261PubMedCrossRefGoogle Scholar
- Carrapiço F (2015) Can we understand evolution without Symbiogenesis? In: Gontier N (ed) Reticulate evolution: Symbiogenesis, lateral gene transfer, hybridization and infectious heredity. Springer, London, pp 81–106CrossRefGoogle Scholar
- Chiu L, Eberl G (2016) Microorganisms as scaffolds of biological individuality: an eco-immunity account of the holobiont. Biol Philos 31:819–837CrossRefGoogle Scholar
- Clarke E (2010) The problem of biological individuality. Biological Theory 5(4):312–325CrossRefGoogle Scholar
- Crombie JM (1886) On the algo-lichen hypothesis. Journal of Linnaean Society 21:259–282CrossRefGoogle Scholar
- Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL (2015) Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347(6218):170–175PubMedPubMedCentralCrossRefGoogle Scholar
- Dawkins R (1976) The Selfish Gene. Oxford, Oxford University PressGoogle Scholar
- De Bary A (1879) Die Erscheinung der Symbiose. Verlag von Karl J, TrübnerGoogle Scholar
- Díaz, JS (2015) El mecanismo evolutivo de Margulis y los niveles de selección. Contrastes: Revista internacional de filosofía 20(1):7–24Google Scholar
- DiFrisco J (2017) Kinds of biological individuals: Sortals, projectability, and selection. Br J Philos SciGoogle Scholar
- Doolittle WF (2017) Darwinizing Gaia. J Theor Biol 434:11–19PubMedCrossRefGoogle Scholar
- Doolittle WF, Booth A (2017) It’s the song not the singer: an exploration of holobiosis and evolutionary theory. Biol Philos 32:5–24. https://doi.org/10.1007/s10539-016-9542-2 CrossRefGoogle Scholar
- Douglas AE (2010) The symbiotic habit. Princeton University Press, OxfordGoogle Scholar
- Douglas AE, Werren JH (2016) Holes in the hologenome: why host-microbe symbioses are not holobionts. MBio 7(2):e02099–e02015PubMedPubMedCentralCrossRefGoogle Scholar
- Dupré J (2010) The polygenomic organism. Sociol Rev 58(s1):19–30CrossRefGoogle Scholar
- Dupré J (2012) Processes of life: essays in the philosophy of biology. Oxford University Press, OxfordCrossRefGoogle Scholar
- Dupré J, O’Malley MA (2009) Varieties of living things: life at the intersection of lineage and metabolism. Philosophy & Theory in Biology 1(December). https://doi.org/10.3998/ptb.6959004.0001.003
- Egerton FN (2015) History of ecological sciences, part 52: Symbiosis studies. Bulletin of Ecological Society of America 96(1):80–139CrossRefGoogle Scholar
- Frank R (1877) Über die biologischen Verthältnisse des Thallus eineger Krustenflecten. Beitrage zur Biologie der Pflanzen 2:123–200Google Scholar
- Frank R (1885) Über die auf Wurzelsymbiose beruhende Ernährung gewisser Bäume durch unterirdische Pilze. Berichte der Deutschen Botanischen Gesellschaf 3:128–145Google Scholar
- Frank R (2005) On the nutritional dependence of certain trees on root symbiosis with belowground fungi (an English translation of a.B. Frank’s classic paper of 1885). Mycorrhiza 15:267–275PubMedCrossRefGoogle Scholar
- Geddes P (1882) Further researchers on animals containing chlorophyll. Nature 25:303–304CrossRefGoogle Scholar
- Gilbert SF, Epel D (2009) Ecological Developmental Biology. Sinauer AssociatesGoogle Scholar
- Gilbert SF, Sapp J, Tauber AI (2012) A symbiotic view of life: we have never been individuals. Q Rev Biol 87(4):325–341PubMedCrossRefPubMedCentralGoogle Scholar
- Gilbert SF, Rosenberg E, Zilber-Rosenberg I (2017) The holobiont with its hologenome is a level of selection in evolution. In: Gissis SB, Lamm E, Shavit A (eds) Landscapes of collectivity in the life sciences. The MIT Press, London, pp 305–324Google Scholar
- Gissis SB, Lamm E, Shavit A (eds) (2017) Landscapes of collectivity in the life sciences. The MIT Press, CambridgeGoogle Scholar
- Godfrey-Smith P (2009) Darwinian populations and natural selection. Oxford University Press, OxfordCrossRefGoogle Scholar
- Godfrey-Smith P (2015) Reproduction, symbiosis, and the eukaryotic cell. PNAS 112(33):10120–10125PubMedCrossRefGoogle Scholar
- Gontier N (2015) Reticulate evolution: Symbiogenesis, lateral gene transfer, hybridization and infectious heredity. Springer, LondonCrossRefGoogle Scholar
- Gontier N (2016a) Symbiosis. In: Kliman RM (ed) The Encyclopaedia of evolutionary biology, vol 4. Academic Press, Oxford, pp 272–281CrossRefGoogle Scholar
- Gontier N (2016b) Symbiogenesis. In: Kliman RM (ed) The Encyclopaedia of evolutionary biology, vol 4. Academic Press, Oxford, pp 261–271CrossRefGoogle Scholar
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O et al (2014) Human genetics shapes the gut microbiome. Cell 159:789–799PubMedPubMedCentralCrossRefGoogle Scholar
- Goodrich JK, Davenport ER, Beaumont M, Clark AG, Ley RE (2017) The relationship between the human genome and microbiome comes into view. Annu Rev Genet 51:413–433PubMedPubMedCentralCrossRefGoogle Scholar
- Griesemer J (2016) Reproduction in complex life cycles: a developmental reaction norms perspective. Philos Sci 83:803–815CrossRefGoogle Scholar
- Griesemer J (2017) Landscapes of developmental collectivity. In: Gissis SB, Lamm E, Shavit A (eds) Landscapes of collectivity in the life sciences. The MIT Press, London, pp 25–48Google Scholar
- Guerrero R, Margulis L, Berlanga M (2013) Symbiogenesis: the holobiont as a unit of evolution. Int Microbiol 16:133–143PubMedGoogle Scholar
- Hester ER, Barott KL, Nulton J, Vermeij MJA, Rohwer FL (2016) Stable and sporadic symbiotic communities of coral and algal holobionts. The ISME Journal 10:1157–1169PubMedCrossRefGoogle Scholar
- Honegger R (2000) Simon Schwendener (1829–1919) and the dual hypothesis of lichens. Bryologist 103(2):307–313. https://doi.org/10.1639/0007-2745(2000)103[0307:SSATDH]2.0.CO;2Google Scholar
- Hull DL (1980) Individuality and selection. Annu Rev Ecol Syst 11:311–332. https://doi.org/10.1146/annurev.es.11.110180.001523 CrossRefGoogle Scholar
- Hurst GDD (2017) Extended genomes: symbiosis and evolution. Interface Focus 7:20170001. https://doi.org/10.1098/rsfs.2017.0001 PubMedPubMedCentralCrossRefGoogle Scholar
- Huttenhower C, Gevers D, Knight R, Creas HH et al (2012) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214CrossRefGoogle Scholar
- Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215PubMedCrossRefGoogle Scholar
- Khakhina LN (1992) Concepts of Symbiogenesis: a historical and critical study of the research of Russian botanists. Yale University Press, New HavenGoogle Scholar
- Kozo-Polyanski M (1924 [2010]) Symbiogenesis. A new principle in evolution. Edited by V Fett & L Margulis. Cambridge, Harvard University PressGoogle Scholar
- Kropoptkin P (1902) Mutual aid. A factor of evolution. William Heinemann, LondonGoogle Scholar
- Lamm E (2017) Cultural group selection and Holobiont evolution: a comparison of structures of evolution. In: Gissis SB, Lamm E, Shavit A (eds) Landscapes of collectivity in the life sciences. The MIT Press, London, pp 369–384Google Scholar
- Lemanceau P, Blouin M, Muller D, Moënne-Loccoz Y (2017) Let the core microbiota be functional. TRENDS in Plant Science 22 (7): 583–595Google Scholar
- Lewontin RC (1970) The units of selection. Annu Rev Ecol Syst 1:1–18CrossRefGoogle Scholar
- Lidgard S, Nyhart LK (2017) The work of biological individuality. Concepts and contexts. In: Lidgard S, Nyharts LK (eds) Biological individuality. Integrating scientific, philosophical and historical perspectives. The University of Chicago Press, London, pp 17–62Google Scholar
- Lipnicki LL (2015) The role of symbiosis in the transmission of some eukaryotes from aquatic to terrestrial environments. Symbiosis 65:39–53CrossRefGoogle Scholar
- Lloyd E (2017a) Units and Levels of selection. In EN Zalta (ed.) Stanford Encyclopaedia of Philosophy. https://plato.stanford.edu/entries/selection-units/
- Lloyd E (2017b) Holobionts as units of selection: Holobionts as interactors, reproducers, and manifestors of adaptation. In: Gissis SB, Lamm E, Shavit A (eds) Landscapes of collectivity in the life sciences. The MIT Press, London, pp 351–367Google Scholar
- Lloyd E (2017c) A glimpse of philosophy of biology and collectivities today. In: Gissis SB, Lamm E, Shavit A (eds) Landscapes of collectivity in the life sciences. The MIT Press, London, pp 291–301Google Scholar
- Margulis L (1970) The origin of eukaryotic cells. Yale University PressGoogle Scholar
- Margulis L (1990) Words as battle cries – symbiogenesis and the new field of endocytobiology. Bio Sci 40(9):673–677Google Scholar
- Margulis L (1991) Symbiogenesis and symbioticism. In: Margulis L, Fester R (eds) Symbiosis as a source of evolutionary innovation. The MIT Press, Cambridge, pp 1–14Google Scholar
- Margulis L (1993) Symbiosis in cell evolution: microbial communities in the Archean and Proterozoic eons. WH Freeman and Co., New YorkGoogle Scholar
- Margulis L (1998) Symbiotic planet. A new look at evolution. Basic Books, New YorkGoogle Scholar
- Margulis L (2010) Symbiogenesis. A new principle in evolution. Paleontol J 44(12):1525–1539CrossRefGoogle Scholar
- Margulis L, Fester R (eds) (1991) Symbiosis as a source of evolutionary innovation. The MIT Press, CambridgeGoogle Scholar
- Margulis L, Sagan D (2002) Acquiring genomes. A theory of the origin of species. Basic Books, New YorkGoogle Scholar
- Martin W, Kowallik K (1999) Annotated English translation of Mereschkowsky’s 1905 paper “Über Natur und Ursprung der Chromatophoren im Pflanzanreiche”. Eur J Phycol 34(3):287–295Google Scholar
- Martin BD, Schwab E (2012) Symbiosis: “living together” in chaos. Studies in the History of Biology 4(4):7–25Google Scholar
- Martin BD, Schwab E (2013) Current usage of symbiosis and associated terminology. International Journal of Biology 5:32–45Google Scholar
- Maynard-Smith J (1987) Evolutionary progress and levels of selection. In: Dupré J (ed) The latest on the best: essays on evolution and optimality. MIT Press, Cambridge, pp 119–131Google Scholar
- Maynard-Smith J (1991) A Darwinian view of symbiosis. In: Margulis L, Fester R (eds) Symbiosis as a source of evolutionary innovation. The MIT Press, Cambridge, pp 26–39Google Scholar
- McFall-Ngai M (2015) Giving microbestheir due – animal life in amicrobially dominant world. J Exp Biol 218:1968–1973PubMedCrossRefGoogle Scholar
- McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, Eberl G et al (2013) Animals in the bacterial world, a new imperative for the life sciences. PNAS 110(9):3229–3236PubMedCrossRefGoogle Scholar
- Merezhkowsky C (1905) Über Natur und Ursprung der Chromatophoren imPflanzanreiche. Biologisches Centralblatt 25:593–604Google Scholar
- Merezhkowsky C (1910) Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen. Biologisches Centralblatt 30:278–303Google Scholar
- Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV et al (2016) Cospeciation of gut microbiota with hominids. Science 353:380–382PubMedPubMedCentralCrossRefGoogle Scholar
- Moran N, Sloan DB (2015) The Hologenome concept: helpful or hollow? PLoSBiol 13(12):e1002311CrossRefGoogle Scholar
- O’Malley MA (2017) From endosymbiosis to holobionts: evaluating a conceptual legacy. J Theor Biol 434:34–41. https://doi.org/10.1016/j.jtbi.2017.03.008 PubMedCrossRefGoogle Scholar
- O’Malley MA, Dupré J (2007) Size doesn’t matter: towards a more inclusive philosophy of biology. Biol Philos 22:155–191CrossRefGoogle Scholar
- Ochman H, Worobey M, Kuo C-H, Ndjango N-BN, Peeters M et al (2010) Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol 8(11):e10000546CrossRefGoogle Scholar
- Okasha S (2006) Evolution and the levels of selection. Oxford University Press, OxfordCrossRefGoogle Scholar
- Oldroyd GED (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263PubMedCrossRefGoogle Scholar
- O'Malley MA (2014) Philosophy of microbiology. Cambridge University Press, CambridgeCrossRefGoogle Scholar
- Oulhen N, Schulz BJ, Carrier TJ (2016) English translation of Heinrich Anton de Bary’s 1878 speech, ‘die Erscheinung der Symbiose’(‘De la symbiose’). Symbiosis 69:131–139. https://doi.org/10.1007/s13199-016-0409-8 CrossRefGoogle Scholar
- Paracer S, Ahmadjian V (2000) Symbiosis: an introduction to biological associations. Oxford University Press, OxfordGoogle Scholar
- Peacock KA (2011) Symbiosis in ecology and evolution. In: Gabbay DM, Thagard P, Woods J (eds) Handbook of the philosophy of science: philosophy of ecology. North Holland, San Diego, pp 219–250CrossRefGoogle Scholar
- Portier P (1918) Les Symbiotes. Masson, ParisGoogle Scholar
- Pound R (1893) Symbiosis and mutualism. Am Nat 27(318):509–520CrossRefGoogle Scholar
- Pradeu T (2016a) The many faces of biological individuality. Biol Philos 31:761–773CrossRefGoogle Scholar
- Pradeu T (2016b) Organisms or biological individuals? Combining physiological and evolutionary individuality. Biol Philos 31:797–817CrossRefGoogle Scholar
- Queller DC, Strassmann JE (2009) Beyond society: the evolution of organismality. Philos Trans R Soc B 364:3143–3155CrossRefGoogle Scholar
- Queller DC, Strassmann JE (2016) Problems of multispecies organisms: endosymbionts to holobionts. Biol Philos 31:855–873CrossRefGoogle Scholar
- Relman DA (2012) Microbiology: learning about who we are. Nature 486:194–195PubMedCrossRefGoogle Scholar
- Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E (2006) The coral probiotic hypothesis. Environ Microbiol 8:2068–2073PubMedCrossRefGoogle Scholar
- Rosas-Pérez T, Vera-Ponce de León A, Ramírez-Puebla ST, Rincón-Rosales R, Martínez-Romer J, Dunn MF, Kondorosi E & Martínez-Romero E (2017) The Symbiome of Llaveia Cochineals (Hemiptera: Coccoidea: Monophlebidae) Includes a Gammaproteobacterial Cosymbiont Sodalis TME1 and the Known Candidatus Walczuchella monophlebidarum. In VDC Shields (ed.): Insect Physiology and Ecology. DOI: https://doi.org/10.5772/66442. Available from: https://mts.intechopen.com/books/insect-physiology-and-ecology/the-symbiome-of-llaveia-cochineals-hemiptera-coccoidea-monophlebidae-includes-a-gammaproteobacterial
- Rosenberg E, Zilber-Rosenberg I (2014) TheHologenome concept. Springer, LondonGoogle Scholar
- Rosenberg E, Zilber-Rosenberg I (2016) Microbes drive evolution of animals and plants: the hologenome concept. MBio 7(2):e01395–e01315PubMedPubMedCentralCrossRefGoogle Scholar
- Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 5:355–362PubMedCrossRefGoogle Scholar
- Rosenberg E, Sharon G, Atad I, Zilber-Rosenberg I (2010) The evolution of animals and plants via symbiosis with microorganisms. Environ Microbiol Rep 2(4):500–506PubMedCrossRefGoogle Scholar
- Roughgarden J, Gilbert SF, Rosenberg E, Zilber-Rosenberg I & Lloyd EA (2017). Holobionts as units of selection and a model of their population dynamics and evolution. Biological Theory Google Scholar
- Sagan L (1967) On the origin of mitosing cells. Journal of Theoretical Biology 14: 225–274Google Scholar
- Sapp J (1994) Evolution by association. A history of symbiosis. Oxford University Press, New YorkGoogle Scholar
- Sapp J (2002) Paul Buchner (1886-1978) and hereditary symbiosis in insects. Int Microbiol 5(3):145–150PubMedCrossRefGoogle Scholar
- Sapp J (2003) Genesis: the evolution of biology. Oxford University Press, New YorkCrossRefGoogle Scholar
- Sapp J (2004) The dynamics of symbiosis: an historical overview. Can J Bot 82:1046–1056CrossRefGoogle Scholar
- Sapp J (2010) Saltational symbiosis. Theory Biosciences 129:125–133CrossRefGoogle Scholar
- Sapp J, Carrapiço F, Zolotonosov M (2002) Symbiogenesis. The hidden face of Constantin Merezhkowky. History and Philosophy of the Life Sciences 24(3/4):413–440PubMedCrossRefGoogle Scholar
- Schneider A (1897) The phenomena of Symbiosis. Minnesota Botanical Studies 1(9):923–948Google Scholar
- Schwendener S (1868) Über die Beziehungen zwischen Algen und Flechtengonidien. Botanische Zeitung [Berlin]: 289–292Google Scholar
- Shropshire JD, Bordenstein SR (2016) Speciation by symbiosis: the microbiome and behavior. MBio 7(2):e01785–e01715PubMedPubMedCentralCrossRefGoogle Scholar
- Skillings D (2016) Holobionts and the ecology of organisms: multi-species communities or integrated individuals? Bio Philos 31:875–892CrossRefGoogle Scholar
- Sommer F, Bäckhed F (2013) The gut microbiota – masters manipulator of host development and physiology. Nat Rev Microbiol 11(4):227–238PubMedCrossRefGoogle Scholar
- Spencer H (1899) The principles of biology. D. Appleton & Co., New YorkCrossRefGoogle Scholar
- Stahl E (1877) Beiträge zur Entwickelungsgeschichte der Flechten (vols. 1 & 2). Leipzig: A FelixGoogle Scholar
- Stencel A (2016) The relativity of Darwinian populations and the ecology of endosymbiosis. BiolPhilos 31:619–637Google Scholar
- Taxis TM, Wolff S, Gregg SJ, Minton NO, Zhang C, Dai J, Schnabel RD, Taylor JF, Kerley MS, Pires JC, Lamberson WR, Conant GC (2015) The players may change but the game remains: network analyses of ruminal microbiomes suggest taxonomic differences mask functional similarity. Nucleic Acids Res 43(20):9600–9612PubMedPubMedCentralGoogle Scholar
- Theis KR, Dheilly NM, Klassen JL, Brucker RM, Baines JF, Bosch TCG, Cryan JF, Gilbert SF, Goodnight CJ, Lloyd EA, Sapp J, Vandenkoornhuyse P, Zilber-Rosenberg I, Rosenberg E, Bordenstein SR (2016) Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems 1(2):e00028–e00016PubMedPubMedCentralCrossRefGoogle Scholar
- Trappe JM (2005) A. B. Frank and mycorrhizae: the challenge to evolutionary and ecologic theory. Mycorrhiza 15(4):277–281PubMedCrossRefGoogle Scholar
- Tripp EA, Zhans N, Schneider H, Huang Y, Mueller GM, Hu Z, Häggblom M, Bhattacharya D (2017) Reshaping Darwin’s tree: impact of the symbiome. TRENDS in Ecology and Evolution 32(8):552–555PubMedCrossRefGoogle Scholar
- Turpin W, Espín-García O, Xu W, Silverberg MS, Kevans D, Smith MI, Guttman DS, Griffiths A et al (2016) Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet 48(11):1413–1417PubMedCrossRefGoogle Scholar
- Van Beneden P-J (1876) Animal parasites and messmates. Henry S. King, LondonCrossRefGoogle Scholar
- Wallin IE (1927) Symbioticism and the origin of species. Williams & Wilkins Co., BaltimoreGoogle Scholar
- Wilkinson DM (2001) At cross purposes. Nature 412:485PubMedCrossRefGoogle Scholar
- Wilson RA, Barker M (2013) The biological notion of individual. In EN Zalta (ed.) The Stanford Encyclopedia of Philosophy. https://plato.stanford.edu/archives/spr2017/entries/biology-individual/
- Zilber-Rosenberg I, Rosenberg E (2008) Role of microorganisms in the evolution of animals and plants: the hologenometheoryof evolution. FEMS Microbiol Rev:723–735Google Scholar
- Zook D (2015) Symbiosis: Evolution’s co-author. In: Gontier N (ed) Reticulate Evolution. Springer, London, pp 41–80CrossRefGoogle Scholar